EO exists naturally and comes from a variety of sources
EO has a variety of modern uses
EO is primarily used to make other chemicals; and EO, and its derivatives, also help make or supply a variety of products we use every day:
EO exists in the air all around us
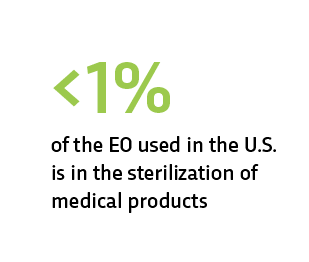
EO is essential to the U.S. health care system
The U.S. Food & Drug Administration (FDA) has stated repeatedly that EO is essential to the U.S. health care system. Hospitals and patients depend on EO sterilization for critical and lifesaving products. Billions of medical products and devices are used every year, and many must be sterile before use to ensure the safety of patients and health care providers.
-
For many medical devices, EO is the only sterilization method that effectively sterilizes without damaging the device during the sterilization process. Medical devices made from certain polymers (plastic or resin) or that have multiple layers of packaging or hard-to-reach places (for example, catheters) are likely to be sterilized with EO.
The method of sterilization for a particular product or device is determined by the medical product manufacturer based on a variety of considerations, including FDA sterility requirements and internationally agreed consensus standards, the materials used and design of the product being sterilized, and the intended use of the product. Without EO sterilization, infection risk associated with surgical procedures and other forms of care could be meaningfully increased.
FDA - 10/25/2019, Press release
“Without adequate availability of ethylene oxide sterilization, we anticipate a national shortage of these devices and other critical devices including feeding tube devices used in neonatal intensive care units, drug-eluting cardiac stents, catheters, shunts and other implantable devices. It’s important to note at this time there are no readily available processes or facilities that can serve as viable alternatives to those that use ethylene oxide to sterilize these devices. In short: this method is critical to our health care system and to the continued availability of safe, effective and high-quality medical devices.”
FDA - 3/20/2020, Press release
“For many medical devices, sterilization with EO may be the only method that effectively sterilizes and does not damage the device during the sterilization process.”
FDA - 8/3/2022, Press release
“While signs of innovation are promising, other methods of sterilization cannot currently replace the use of EtO for many devices. To that end, we are equally concerned about the potential impact of shortages of sterilized medical devices that would result from disruptions in commercial sterilizer facility operations.”