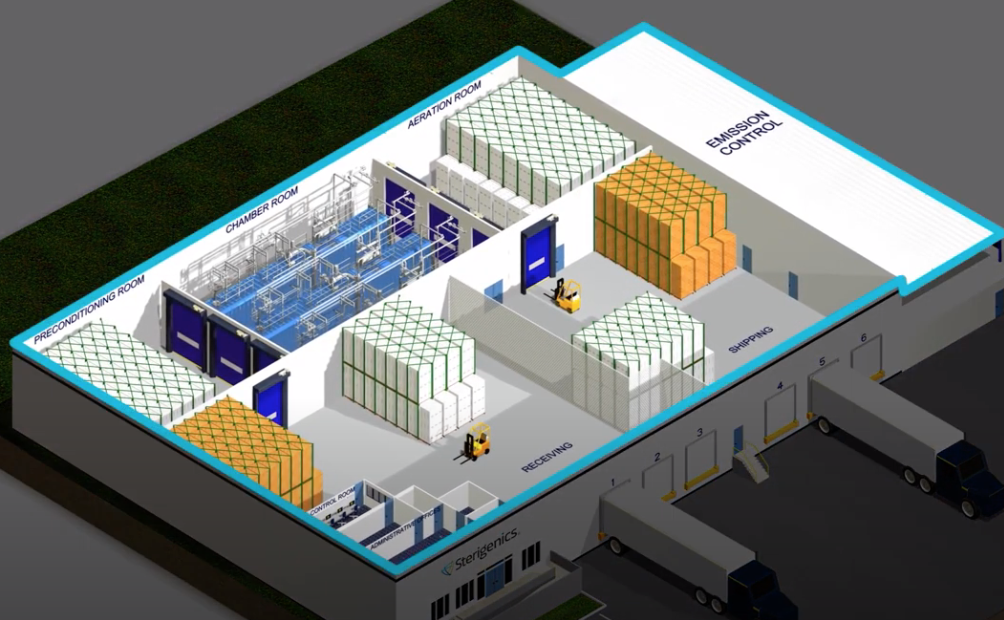
Sterigenics’ Vernon facilities play a critical role in safeguarding public health by performing FDA-approved ethylene oxide sterilization processes on medical equipment prior to use.
Sterilization prevents biological contamination in health care settings that can lead to patient infections, and in severe cases, deaths.
For many medical devices, sterilization with EO is the only method that effectively sterilizes and does not damage the device during the sterilization process. The Vernon facilities sterilize over 45 million essential medical devices and supplies each year, including surgical kits, catheters, cardiac implants, stents, IV sets and more.
These products are supplied to nearly 100 healthcare product manufacturers, including dozens in the Los Angeles-area, as well as local hospitals.
The facilities also provide over 40 well-paying industrial jobs in the area.